GAMMAGARD LIQUID [Immune Globulin Infusion (Human)] 10% Solution is indicated as a replacement therapy for primary humoral immunodeficiency (PI) in adult and pediatric patients ≥2 years.
Takeda’s Commitment

Who manufactures GAMMAGARD LIQUID?
GAMMAGARD LIQUID is manufactured by Takeda, a global, values-based, research- and development-driven biopharmaceutical leader headquartered in Japan. Takeda is committed to patients by translating science into highly innovative medicines.
As a reliable source of immune globulin (IG) therapies for over 30 years, GAMMAGARD LIQUID is one of five IG therapies that Takeda has available.1
Check out this video to learn more about how GAMMAGARD LIQUID is made
Examine the creation of GAMMAGARD LIQUID, from the donor selection process to purification and the dedicated virus inactivation steps.
Before we get started, let’s go through some important safety information. GAMMAGARD LIQUID is indicated as a replacement therapy for primary humoral immunodeficiency (Pl) in adult and pediatric patients two years of age or older, as a maintenance therapy to improve muscle strength and disability in adult patients with Multifocal Motor Neuropathy (MMN), and as a therapy to improve neuromuscular disability and impairment in adult patients with Chronic Inflammatory Demyelinating Polyneuropathy (CIDP). LIMITATIONS OF USE (CIDP): GAMMAGARD LIQUID has not been studied in immunoglobulin-naive patients with CIDP. GAMMAGARD LIQUID maintenance therapy in CIDP has not been studied for periods longer than 6 months. After responding during an initial treatment period, not all patients require indefinite maintenance therapy with GAMMAGARD LIQUID in order to remain free of CIDP symptoms. Individualize the duration of any treatment beyond 6 months based upon the patient’s response and demonstrated need for continued therapy. GAMMAGARD LIQUID for PI is for intravenous or subcutaneous use. GAMMAGARD LIQUID for MMN and CIDP is for intravenous use only.
IMPORTANT SAFETY INFORMATION WARNING: THROMBOSIS, RENAL DYSFUNCTION, and ACUTE RENAL FAILURE Thrombosis may occur with immune globulin (IG) products, including GAMMAGARD LIQUID. Risk factors may include advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling vascular catheters, hyperviscosity, and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors. Renal dysfunction, acute renal failure, osmotic nephrosis, and death may occur in predisposed patients with immune globulin intravenous (IGIV) products. Patients predisposed to renal dysfunction include those with any degree of pre-existing renal insufficiency, diabetes mellitus, age greater than 65, volume depletion, sepsis, paraproteinemia, or patients receiving known nephrotoxic drugs. Renal dysfunction and acute renal failure occur more commonly in patients receiving IGIV products containing sucrose. GAMMAGARD LIQUID does not contain sucrose. For patients at risk of thrombosis, administer GAMMAGARD LIQUID at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk of hyperviscosity. Please see additional Important Safety Information in this video, and the accompanying Full Prescribing Information.
Want to see one of the world’s most precious assets?
It’s not a diamond, it's not gold.
This is human plasma, and it’s used to create immunoglobulin therapies, like GAMMAGARD LIQUID.
Pretty cool, huh?
But do you know how these therapies are made? Let me show you.
Precious things need to be handled with care. And this is no exception.
It’s not a process that can be rushed. No, no.
It’s a journey of dedication and care.
The process takes up to a year and follows rigorous quality and safety measures.
It begins with donor selection.
Potential donors are strictly screened before their plasma is collected and their donation is tested for viruses and infections.
Then, experts carefully remove the IgG antibodies that are needed to create the final product. After this, they purify the antibodies IgG antibodies in several stages.
And, while donor screening and donation testing minimize the chances of viruses, three specialized steps clear any viruses that may still be there.
Immunoglobulin therapies are all produced in a similar way, but their features are different.
GAMMAGARD LIQUID is designed with patient needs in mind.
Its specific characteristics make it suitable for a broad range of patients.
For example, it doesn’t contain any added sugar or sodium, which may be important things to consider for patients who are watching their salt and sugar intake.
Looking good!
After a final inspection, GAMMAGARD LIQUID is shipped to the people it’s prescribed to.
People like me!
INDICATIONS
GAMMAGARD LIQUID is indicated as a replacement therapy for primary humoral immunodeficiency (Pl) in adult and pediatric patients two years of age or older, as a maintenance therapy to improve muscle strength and disability in adult patients with Multifocal Motor Neuropathy (MMN), and as a therapy to improve neuromuscular disability and impairment in adult patients with Chronic Inflammatory Demyelinating Polyneuropathy (CIDP).
LIMITATIONS OF USE (CIDP): GAMMAGARD LIQUID has not been studied in immunoglobulin-naïve patients with CIDP. GAMMAGARD LIQUID maintenance therapy in CIDP has not been studied for periods longer than 6 months. After responding during an initial treatment period, not all patients require indefinite maintenance therapy with GAMMAGARD LIQUID in order to remain free of CIDP symptoms.
Individualize the duration of any treatment beyond 6 months based upon the patient's response and demonstrated need for continued therapy.
GAMMAGARD LIQUID for PI is for intravenous or subcutaneous use.
GAMMAGARD LIQUID for MMN and CIDP is for intravenous use only.
IMPORTANT SAFETY INFORMATION
WARNING: THROMBOSIS, RENAL DYSFUNCTION, and ACUTE RENAL FAILURE.
- Thrombosis may occur with immune globulin (IG) products, including GAMMAGARD LIQUID. Risk factors may include advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling vascular catheters, hyperviscosity, and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors.
- Renal dysfunction, acute renal failure, osmotic nephrosis, and death may occur in predisposed patients with immune globulin intravenous (IGIV) products. Patients predisposed to renal dysfunction include those with any degree of pre-existing renal insufficiency, diabetes mellitus, age greater than 65, volume depletion, sepsis, paraproteinemia, or patients receiving known nephrotoxic drugs. Renal dysfunction and acute renal failure occur more commonly in patients receiving IGIV products containing sucrose. GAMMAGARD LIQUID does not contain sucrose.
- For patients at risk of thrombosis, administer GAMMAGARD LIQUID at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk of hyperviscosity.
CONTRAINDICATIONS
- History of anaphylactic or severe systemic hypersensitivity reactions to human IG.
- IgA-deficient patients with antibodies to IgA and a history of hypersensitivity to human IG. Anaphylaxis has been reported with intravenous (IV) use of GAMMAGARD LIQUID.
WARNINGS AND PRECAUTIONS
Hypersensitivity: Severe hypersensitivity reactions may occur, even in patients who have tolerated previous treatment with human IG. If a hypersensitivity reaction occurs, discontinue infusion immediately and institute appropriate treatment. IgA-deficient patients with antibodies to IgA are at greater risk of developing potentially severe hypersensitivity reactions, including anaphylaxis.
Renal Dysfunction/Failure: Acute renal dysfunction/failure, acute tubular necrosis, proximal tubular nephropathy, osmotic nephrosis, and death may occur with IV use of IG products, especially those containing sucrose. Acute renal dysfunction/failure has been reported in association with infusions of GAMMAGARD LIQUID. Ensure patients are not volume depleted prior to infusion. In patients at risk due to pre-existing renal insufficiency or predisposition to acute renal failure, assess renal function before initiation and throughout treatment, and use the minimum infusion rate practicable for IV administration. If renal function deteriorates, consider discontinuation.
Hyperproteinemia, increased serum viscosity, and hyponatremia may occur. It is critical to distinguish true hyponatremia from a pseudohyponatremia because certain treatments may lead to volume depletion, a further increase in serum viscosity, and a predisposition to thromboembolic events.
Thrombosis: May occur following treatment with IG products and in the absence of known risk factors. In patients at risk, administer at the minimum dose and infusion rate practicable. Ensure adequate hydration before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity.
Aseptic Meningitis Syndrome: Has been reported with use of IG and may occur more frequently in females. Conduct a thorough neurological exam on patients exhibiting signs and symptoms, to rule out other causes of meningitis. Discontinuing IG treatment has resulted in remission within several days without sequelae.
Hemolysis: GAMMAGARD LIQUID contains blood group antibodies, which may cause a positive direct antiglobulin reaction and hemolysis. Monitor patients for signs and symptoms of hemolysis and delayed hemolytic anemia and, if present, perform appropriate confirmatory lab testing.
Transfusion-Related Acute Lung Injury: Non-cardiogenic pulmonary edema has been reported with IV-administered IG, including GAMMAGARD LIQUID. Monitor patients for pulmonary adverse reactions. If suspected, perform appropriate tests for presence of anti-neutrophil and anti-HLA antibodies in both product and patient serum. May be managed using oxygen therapy with adequate ventilatory support.
Transmittable Infectious Agents: Because GAMMAGARD LIQUID is made from human plasma, it may carry a risk of transmitting infectious agents (e.g., viruses, other pathogens). No confirmed cases of viral transmission or variant Creutzfeldt-Jakob disease (vCJD) have been associated with GAMMAGARD LIQUID.
Interference with Lab Tests: False positive serological test results and certain assay readings, with the potential for misleading interpretation, may occur as the result of passively transferred antibodies.
ADVERSE REACTIONS
The serious adverse reactions observed in clinical studies in PI was aseptic meningitis, and in MMN were pulmonary embolism and blurred vision.
The most common adverse reactions observed in ≥5% of patients were:
IV administration for PI: Headache, fatigue, pyrexia, nausea, chills, rigors, pain in extremity, diarrhea, migraine, dizziness, vomiting, cough, urticaria, asthma, pharyngolaryngeal pain, rash, arthralgia, myalgia, oedema peripheral, pruritus, and cardiac murmur.
Subcutaneous administration for PI: Infusion site (local) event (rash, erythema, edema, hemorrhage, and irritation), headache, fatigue, heart rate increased, pyrexia, abdominal pain upper, nausea, vomiting, asthma, blood pressure systolic increased, diarrhea, ear pain, aphthous stomatitis, migraine, oropharyngeal pain, and pain in extremity.
IV administration for MMN: Headache, chest discomfort, muscle spasms, muscular weakness, nausea, oropharyngeal pain, and pain in extremity.
IV administration for CIDP: Headache, pyrexia, anemia, leukopenia, neutropenia, illness, blood creatinine increased, dizziness, migraine, somnolence, tremor, nasal dryness, abdominal pain upper, vomiting, chills, nasopharyngitis, and pain in extremity.
DRUG INTERACTIONS
Passive transfer of antibodies may transiently interfere with immune responses to live attenuated virus vaccines (e.g., measles, mumps, rubella, and varicella).
Please see the accompanying Full Prescribing Information provided with this material, or on the GAMMAGARD LIQUID website www.gammagard.com/hcp.
Takeda’s Legacy
Takeda's IG Therapies are built on more than 60 years of global heritage in plasma-based therapies.2 Thanks to decades of innovation and an unwavering commitment to providing individualized care, today Takeda offers five IG therapies.
Baxter is founded and paves the way for Takeda's IG portfolio3
PLASMA-VAC is introduced, allowing the separation and storage of plasma from whole blood for the first time3
GAMMABULIN is approved and we begin manufacturing plasma therapy1
GAMMAGARD S/D* [Immune Globulin Intravenous (Human)], IgA less than 1 μg/mL in a 5% solution is approved1
- GAMMAGARD LIQUID is approved for patients aged 2+ with PI1,4
- GAMMAGARD LIQUID is the first IG manufactured with three dedicated virus inactivation/removal steps, increased safety margins5
GAMMAGARD LIQUID is indicated for adults with MMN4,6
HyQvia* [Immune Globulin Infusion (Human), 10% with Recombinant Human Hyaluronidase] Solution is approved for adults with PI1,7
Baxalta is formed as a new independent biopharmaceutical company8
- Cuvitru* [Immune Globulin Subcutaneous (Human)] 20% Solution is approved for patients aged 2+ with PI1,9
- Baxalta becomes part of Shire, expanding global reach10
Takeda acquires Shire and Baxalta, opens new manufacturing facility in Covington, GA11,12
HyQvia* [Immune Globulin Infusion (Human), 10% with Recombinant Human Hyaluronidase] Solution is approved for patients aged 2+ with PI1,7
- HyQvia* [Immune Globulin Infusion (Human), 10% with Recombinant Human Hyaluronidase] Solution gains additional indication for adults with CIDP1,7
- GAMMAGARD LIQUID indicated for maintenance therapy in adults with CIDP. See Limitations of Use (CIDP).1,7
- GAMMAGARD LIQUID ERC [Immune Globulin Infusion (Human)] with less than or equal to 2 µg/mL IgA in a 10% Solution is approved for people aged two and older with PI16
*Please click for Full Prescribing Information, including Boxed Warnings regarding Thrombosis for CUVITRU, HYQVIA, GAMMAGARD LIQUID, GAMMAGARD LIQUID ERC and GAMMAGARD S/D and Renal Dysfunction, and Acute Renal Failure, for GAMMAGARD LIQUID, GAMMAGARD LIQUID ERC, and GAMMAGARD S/D.
Takeda operates 100+ plasma donation centers
With more than 100 state-of-the-art plasma donation centers, BioLife is one of the largest plasma collection networks in the United States.12
Safety standards
Takeda has world-class safety capabilities and an unsurpassed reputation in both plasma donation and pathogen safety. Every step of the process, from collection to manufacturing and delivery, ensures a high-quality product.4,5
Donation safety standards
Strict donation criteria and screening at each visit
Donation frequency management system
Strong inspection record
Plasma screening, inventory hold and look back procedure
Every plasma donation is screened for HIV, hepatitis A, B, and C, and parvovirus B19
Pathogen safety standards
Biosafety level 3+ lab
Purpose-built, state-of-the-art biocontainment laboratory
Process sciences
Fully validated bioprocessing steps
Virology
Classical and molecular virology expertise and capability
Product quality
The manufacturing process of GAMMAGARD LIQUID is designed to ensure reliable, well-controlled, and high safety margins against lipid-enveloped and non-lipid-enveloped viruses.4,5
This includes three dedicated, independent, and effective viral inactivation and removal steps.4,5†
- Step 1
- Step 2
- Step 3
Solvent/detergent treatment5
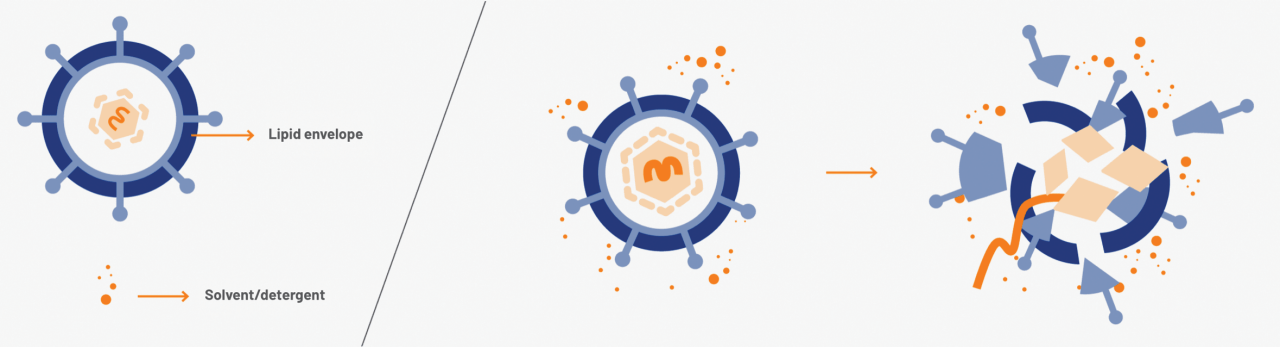
Inactivates lipid-enveloped viruses (eg, HIV) within minutes5
HIV=human immunodeficiency virus.
†Dedicated steps are in addition to fractionation process, and donor and plasma quality measures (plasmapheresis centers are IQPP- and QSEAL-certified).14,15
35 nm nanofiltration5
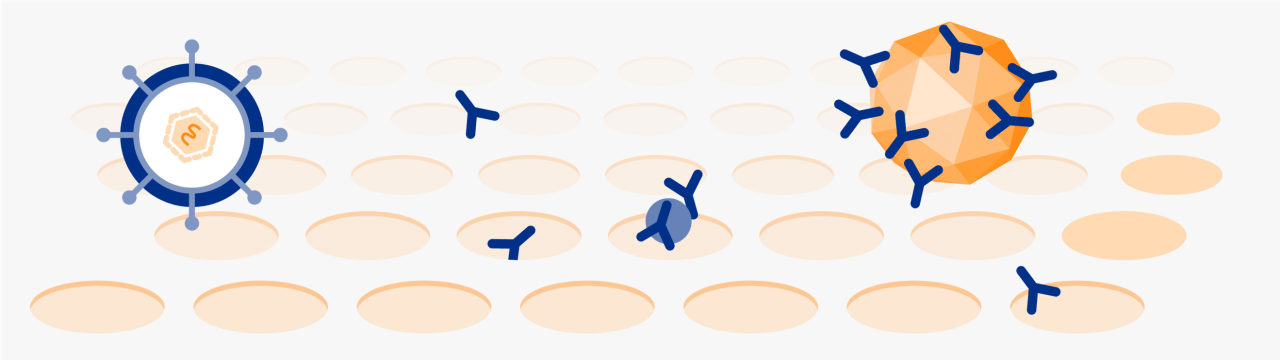
Removes small pathogens by size, which is effective for both lipid-enveloped and relevant non-enveloped (B19V, HAV) viruses4,5
B19V=human parvovirus B19;
HAV=hepatitis A virus.
†Dedicated steps are in addition to fractionation process, and donor and plasma quality measures (plasmapheresis centers are IQPP- and QSEAL-certified).13,14
Incubation at low pH/elevated temperatures5
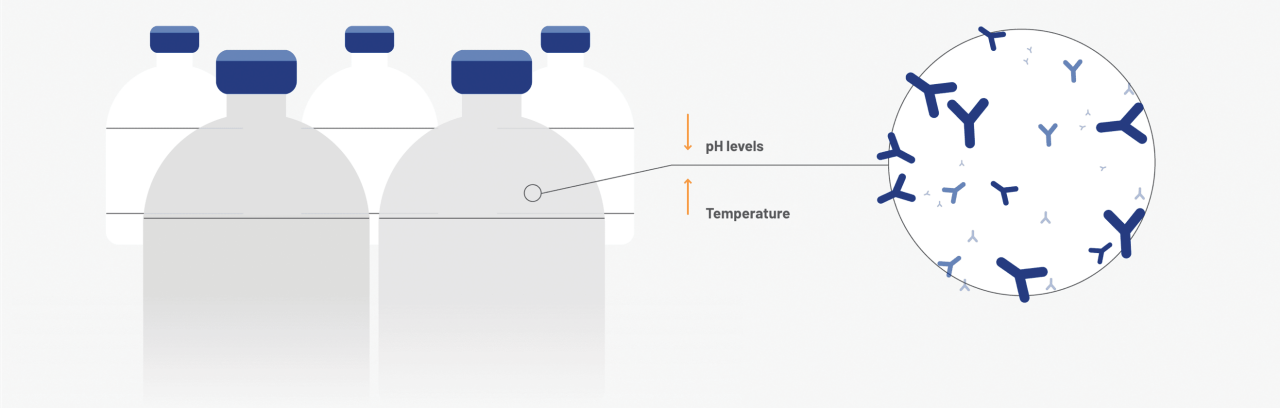
Completely inactivates lipid-enveloped viruses (eg, HIV and HBV/HCV models). Partially effective against non-enveloped viruses (eg, HAV)4,5
HAV=hepatitis A virus; HIV=human immunodeficiency virus; HBV=hepatitis B virus; HCV=hepatitis C virus.
†Dedicated steps are in addition to fractionation process, and donor and plasma quality measures (plasmapheresis centers are IQPP- and QSEAL-certified).13,14
Connect with us for additional information that could improve patient management
Get information regarding GAMMAGARD LIQUID.
References:
- Center for Biologics Evaluation and Research. List of licensed biological products with reference product exclusivity and biosimilarity or interchangeability evaluations to date. Accessed March 13, 2025. https://purplebooksearch.fda.gov/
- Italy CMG 1954 Approval Letter, Gamma Globulin.
- Our history. Baxter. Accessed March 13, 2025. https://www.baxter.com/our-story/our-history
- GAMMAGARD LIQUID. Prescribing Information. Takeda Pharmaceuticals U.S.A., Inc.; 2024.
- Poelsler G, Berting A, Kindermann J, et al. A new liquid intravenous immunoglobulin with three dedicated virus reduction steps: virus and prion reduction capacity. Vox Sang. 2008;94:184-192.
- US Food and Drug Administration. GAMMAGARD LIQUID multifocal motor neuropathy approval letter. 2012.
- HyQvia. Prescribing Information. Takeda Pharmaceuticals U.S.A., Inc.; 2025.
- Sachdev A. Baxter splits into 2 companies. Chicago Tribune. July 1, 2016. Accessed March 13, 2025. https://www.chicagotribune.com/business/ct-baxter-baxalta-split-0702-biz-20150701-story.html
- CUVITRU. Prescribing Information. Takeda Pharmaceuticals U.S.A., Inc.; 2024.
- Shire completes combination with Baxalta creating the global leader in rare diseases and highly specialized conditions. News release. PR Newswire. June 3, 2016. Accessed March 13, 2025. https://www.prnewswire.com/news-releases/shire-completes-combination-with-baxalta-creating-the-global-leader-in-rare-diseases-and-highly-specialized-conditions-581747521.html
- Takeda, Inc. Annual report on Form 20-F for FY2019. June 24, 2020. Accessed July 10, 2025. http://www.takeda.com/4ab333/siteassets/system/investors/report/sec-filings/20-f_2020-06-24.pdf
- Data on file. Covington, GA. Shire US.
- Marketing Research Bureau. The Plasma Proteins Market in the United States (based on aggregated data)–2005-2019.
- Plasma Protein Therapeutics Association. International Quality Plasma Program (IQPP). Accessed March 13, 2025. https://www.pptaglobal.org/material/international-quality-plasma-program-iqpp
- Plasma Protein Therapeutics Association. Quality Standards of Excellence, Assurance and Leadership (QSEAL). Accessed March 13, 2025. https://www.pptaglobal.org/material/quality-standards-of-excellence-assurance-and-leadership-qseal
- GAMMAGARD LIQUID ERC. Prescribing Information. Takeda Pharmaceuticals U.S.A., Inc.; 2025.